Abstract
Background: Somatic mutations in splicing factor 3B subunit 1 (SF3B1) occur in 25% of all myelodysplastic syndromes (MDS) cases and identify a condition characterized by ring sideroblasts, ineffective erythropoiesis, and indolent disease course in lower-risk (LR) MDS. The impact of SF3B1 mutations on erythroid dysregulation has become apparent but little is known about cellular immune phenotypes. Identification of patient characteristics downstream of the pathogenic mutations in SF3B1 may reveal immunological determinants of disease phenotype and response to therapeutic interventions (e.g. luspatercept). In the present study, we applied multiplex immunophenotyping technologies in combination with artificial intelligence (AI)-based analytical approaches to identify genotype-immunophenotype correlations that may affect disease course and clinical outcomes in the SF3B1-MDS subtype.
Methods: We performed transcriptomic immune profiling on bone marrow (BM) mononuclear cells (MNCs) from 12 SF3B1-MUT and 10 SF3B1-WT LR-MDS patients using the NanoString nCounter PanCancer Immune Profiling Panel. Besides, viably frozen PBMC samples (8 SF3B1-MUT; 4 SF3B1-WT) were thawed and stained for CyTOF with a 35-marker MaxPar Immune Profiling Panel (Fluidigm) for deep immune profiling. CyTOF data were analyzed with an in-house developed pipeline (ImmunoCluster, Opzoomer et al. 2021) incorporating the FlowSOM algorithm for unsupervised clustering (K=70). We also retrieved flow cytometric data on fresh BM and peripheral blood (PB) samples acquired independently as part of the diagnostic work-up and re-analyzed them using the T-REX pipeline (Barone and Paul et al. 2020).
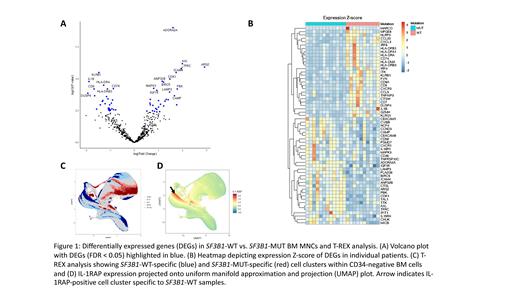
Results: On the transcriptomic level, the SF3B1-MUT subtype showed a distinct inflammatory signature compared to SF3B1-WT (59 DE genes at FDR < 0.05, Fig. 1A & B). GO/KEGG pathway analysis indicated that downregulated genes in SF3B1-MUT patients are involved in antigen processing/presentation (HLA class II transcript, CD8a), regulation of proinflammatory interleukin-1 beta secretion (IL1B, NLRP3), and cellular response to interferon-gamma (HLA class II transcripts, IRF4/8, CCL5, CCL20). Genes that were upregulated in SF3B1-MUT BM MNCs are involved in cell cycle (CDK1, CCND3), proliferation/survival (AXL), iron homeostasis (TFRC), and erythroid differentiation (TAL1).
Unbiased interrogation of cellular phenotypes in PB through FlowSOM analysis of CyTOF data revealed an increased abundance of a CD4 + T cell metacluster resembling a central memory (CM) phenotype in SF3B1-MUT compared to SF3B1-WT PBMCs (7.9 vs 4.5% of CD45 +). Independent automated T-REX-based analysis of clinical flow data supported this finding and further indicated expansion of a cluster resembling CM CD8 + T cells in SF3B1-MUT PB.
Lower IL1B expression in SF3B1-MUT and the implication of the IL-1/IL-1RAP axis in the inflammatory leukemic niche (De Boer et al. 2020) prompted us to re-analyze clinical flow cytometric data on IL-1RAP expression using the T-REX pipeline. T-REX revealed differences in IL-1RAP-expressing clusters between SF3B1-MUT and SF3B1-WT BM samples (9 SF3B1-MUT; 9 SF3B1-WT). Specifically, we noticed a cluster of IL-1RAP + CD34 - cells (HLA-DR + CD123 +) present in SF3B1-WT but greatly contracted in SF3B1-MUT MDS (Fig. 1C & D). Our data suggest that proinflammatory signaling via the IL-1/IL-1RAP axis is reduced in SF3B1-MUT BM.
Aberrant marker expression on SF3B1-MUT monocytes has been reported (Duetz et al. 2020). T-REX analysis of BM CD14 + cells revealed distinct monocyte clusters with differential expression of CD123, CD11b, and HLA-DR. Our initial analysis showed that SF3B1-MUT-specific clusters displayed lower expression of CD123 and CD11b, suggesting that mutations in SF3B1 directly or indirectly affect monocyte phenotypes and most likely function.
Conclusions: We provide evidence that the SF3B1-MDS subtype is associated with a distinct inflammatory signature characterized by lower IL1B expression, changed IL-1RAP + clusters, distinct monocyte phenotypes, and preservation of T cell homeostasis. Further investigation of the immune signature in SF3B1-mutated MDS subtype may lead to using personalized immunotherapy strategies in the future. This work also exemplifies the potential of unsupervised strategies for interrogating cellular immune phenotypes in MDS subtypes within clinical flow cytometry datasets.
Harrison: BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sierra Oncology: Honoraria; Constellation Pharmaceuticals: Research Funding; AOP Orphan Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Promedior: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Galacteo: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Geron: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Keros: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte Corporation: Speakers Bureau; Abbvie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Shire: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead Sciences: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CTI BioPharma: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Platzbecker: Celgene/BMS: Honoraria; Janssen: Honoraria; Novartis: Honoraria; AbbVie: Honoraria; Geron: Honoraria; Takeda: Honoraria. Kordasti: Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Research Funding; Alexion: Honoraria; Beckman Coulter: Honoraria.